Oncology Pharmacy & Chemotherapy Admixing Program
NHBC
TRAINING INSTITUTE
Advancing Chemotherapy Safety Through Specialized Oncology Pharmacy Training


Accredited CPD Provider
Get CPD Points As You Enhance Your Skills for Optimal Resourcefulness
Gain Interactive Training
Course assessments and case studies
NHBC Certificate Issuance
Practice the knowledge you've gained
Chemotherapy medicines are high-risk agents requiring advanced technical competence, strict aseptic practices, and embedded quality assurance systems. Errors at any stage — prescribing, verification, preparation, or handling — can result in serious patient harm and occupational exposure.
NHBC Training Institute was established to strengthen oncology pharmacy practice by developing professionals who build quality and safety into chemotherapy services from the start.
Pharmacists
Pharmaceutical Technologists
17 structured modules
Part of your Continuing Medical Education (CME) journey

Foundational principles of cancer treatment, mechanisms of action of chemotherapy, and the role of pharmacy professionals in oncology care.
Classification of cytotoxic and targeted agents, pharmacological properties, toxicity profiles, and handling considerations.
Principles of chemotherapy dosing, body surface area calculations, dose adjustments, scheduling cycles, and clinical implications of dosing errors.
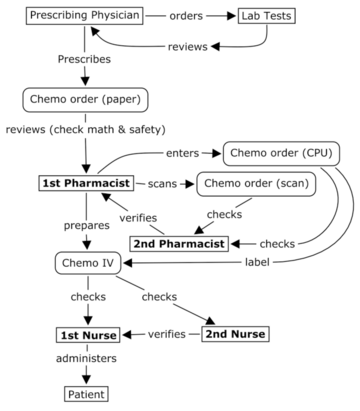
Use of pharmacy workcards for chemotherapy preparation, verification processes, documentation, and traceability.
Pharmacological and supportive management of chemotherapy-induced adverse effects, with emphasis on patient safety and continuity of care.
Proper labelling techniques,
IV priming in the chemo room &
Using closed systems for chemo mixing
Labelling standards for cytotoxic preparations, legal requirements, error prevention, and patient safety considerations. Learn principles and techniques of IV priming, risk reduction strategies, and contamination prevention in chemotherapy environments.
Types of BSCs, certification requirements, airflow principles, correct operational practices, and common safety failures.



Design and organization of chemotherapy rooms, personnel flow, material flow, and contamination control strategies.
Overview of standard chemotherapy regimens, interpretation of protocols, and the pharmacist’s role in regimen selection support.
Rationale, benefits, risks, and clinical considerations when selecting combination or monotherapy regimens.
Safe segregation, handling, and disposal of cytotoxic waste and expired products in compliance with safety and environmental standards.
Design, review, implementation, and updating of SOPs for chemotherapy preparation and oncology pharmacy operations.
Occupational exposure risks, spill management, exposure reporting systems, and health surveillance programs.
Pharmacological principles in palliative oncology care, symptom control, and ethical considerations.
(Four Sub-Modules)
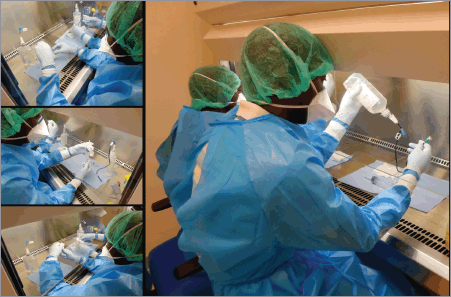
Principles of aseptic compounding
Step-by-step chemotherapy reconstitution
Error prevention during mixing
Quality checks prior to release
(This is the technical core of the program.)
Pharmacy involvement in oncology clinical research, protocol compliance, documentation, and best practices.

